Cómo saber si una molécula es polar o no polar La Ciencia y la
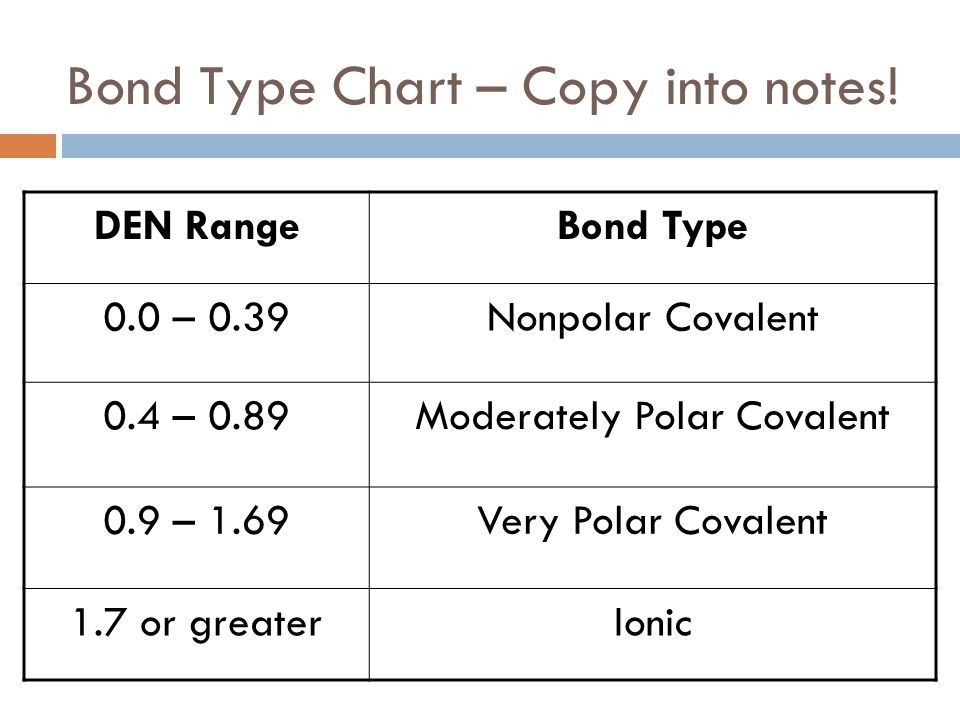
Ionic Polar Covalent Nonpolar Covalent Chart
Is the molecule O C l 2 polar or nonpolar? Explain. Dichlorine Monoxide: Dichlorine monoxide, also called chlorine monoxide, is a brownish-yellow color gas. It is soluble in water and in.
[Solved] image attached 1. Complete the table below. Indicate whether
Question: and VSEPR structure for OCl2 A. Give the electron geometry, (Select] B. Give the bond angle. (Select) C. Give the molecular geometry (Select) D. Does it have polar covalent bonds? (Select) E Is the molecule polar or nonpolar? [Select) F. List all the intermolecular forces present. (Select) G. Give the strongest intermolecular force.

Polar vs. Nonpolar Bonds — Overview & Examples Expii Ionic Bonding
Science Chemistry Chemistry questions and answers Determine whether the following are polar or non-polar: 1. CF3Cl : polar 2. C2H2 : non-polar 3. SF2Cl2 : polar 4. H2O2 : non-polar 5. OCl2 : polar 6. PCl4 : non-polar 7. PO43− : non-polar This problem has been solved!

Is OCl2 Polar or Nonpolar (Dichlorine Monoxide) YouTube
This article explain drawing Cl 2 O Lewis structure, shape, formal charge, resonance in Cl 2 O. As next part, the bond angle, octet rule, Cl 2 O Lewis structure lone pairs of Valence electron, covalent nature, hybridization, solubility, and Cl 2 O polarity also being elaborated.. Dichlorine monoxide is the chemical name for Cl 2 O. Oxygen atom has got two chlorine atoms around it in Cl 2 O.

Polare und unpolare Moleküle Free Press
OCL2, or chlorine (I) oxide, is a chemical compound composed of 1 chlorine atom and one oxygen atom. Its chemical component is Cl2O, a yellowish-inexperienced gas at room temperature. The molecule has a dishonest shape, resulting in its polar nature.
Polar vs Nonpolar bonds What is the Main Difference? PSIBERG
Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole moment that makes the chlorine molecule nonpolar. Chlorine is a highly reactive element and.

Covalent Bonds Biology for Majors I
Dichlorine monoxide (OCl2) is an inorganic compound that is brown-yellow gas at room temperature. It has a molecular weight of 86.9054 g/mol. It has a melting point of -120.6℃ and a boiling point of 2.0℃. Belonging to the chlorine oxide family, OCl2 is soluble in both water and inorganic solvents.

Lewis Structure For Ocl2
Oxygen dichloride has the chemical formula OCl 2 with a molar mass of 86.9054 g/mol. It appears as brownish-yellow gas. It is soluble in water. In this article, we will discuss OCl 2 lewis structure, molecular geometry, bond angle, polar or nonpolar, its hybridization, etc. Oxygen dichloride is a member of the chlorine oxide family of compounds.
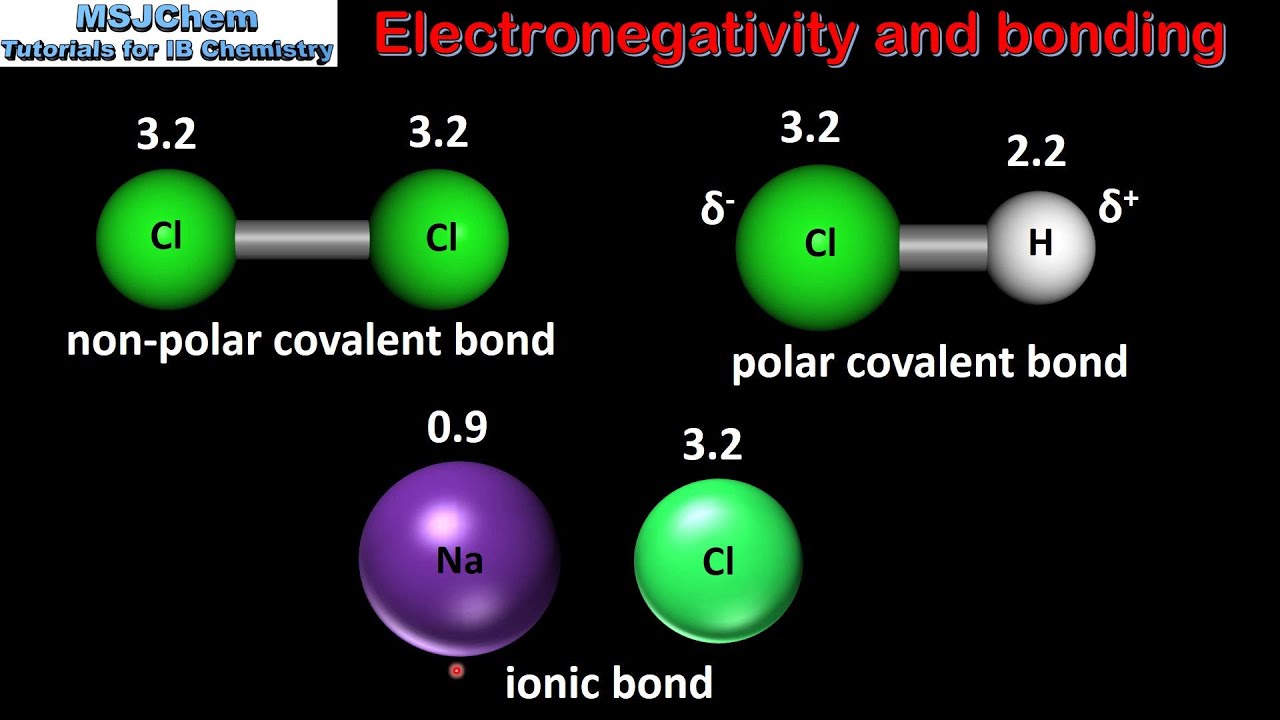
Difference between polar and nonpolar examples
Dichlorine monoxide is an inorganic compound with the molecular formula Cl 2 O. It was first synthesised in 1834 by Antoine Jérôme Balard, [2] who along with Gay-Lussac also determined its composition.
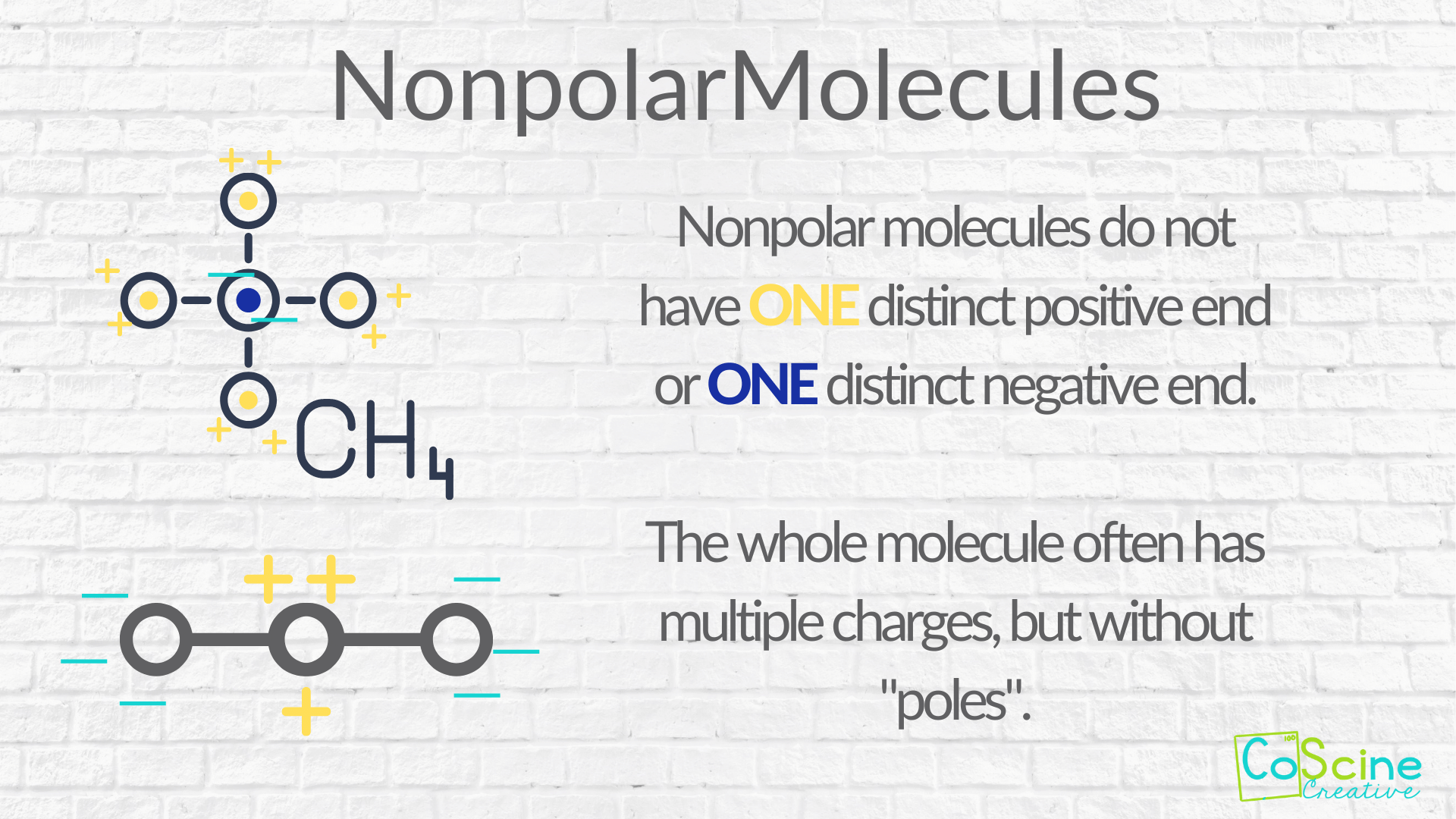
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative
By Triyasha Mondal Ocl2 lewis structure shows the bonding structure of involving atoms in the molecule. The article will discuss about it briefly. Outer shell electrons of the involved atoms are shown in the ocl2 lewis structure. These electrons effect the properties of the molecule.
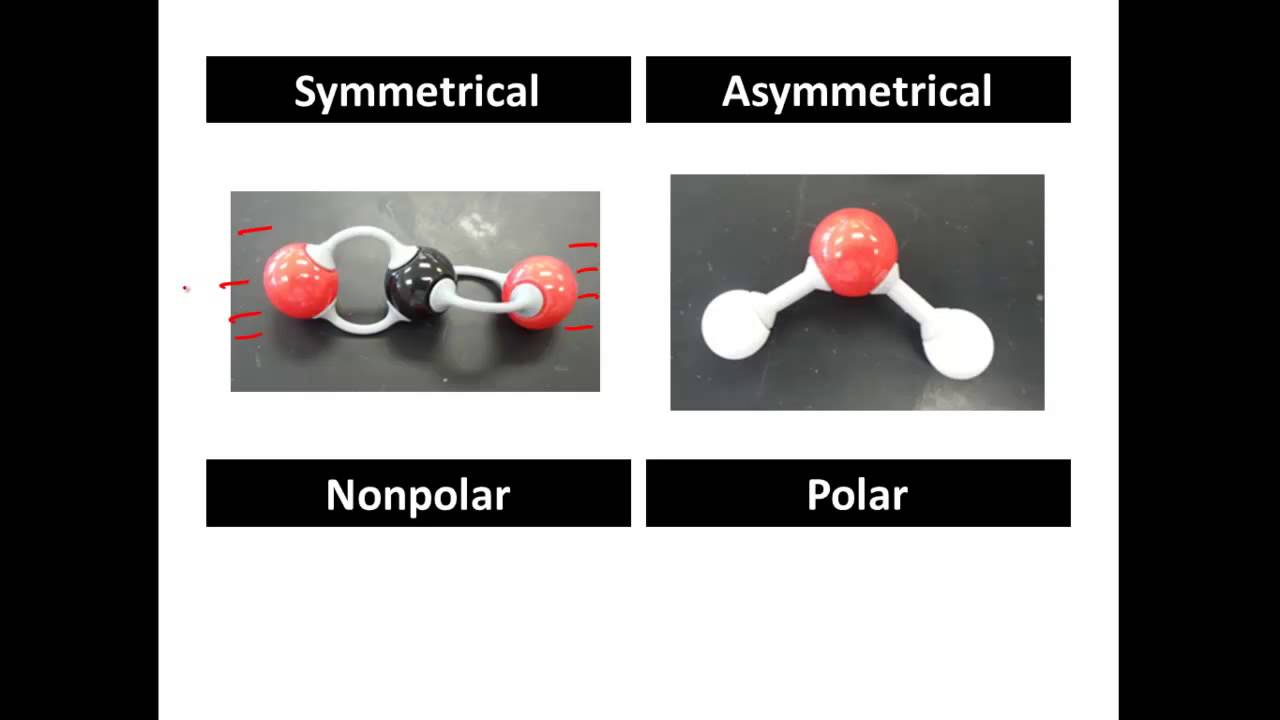
Polar and Nonpolar Molecules JourneyilSalinas
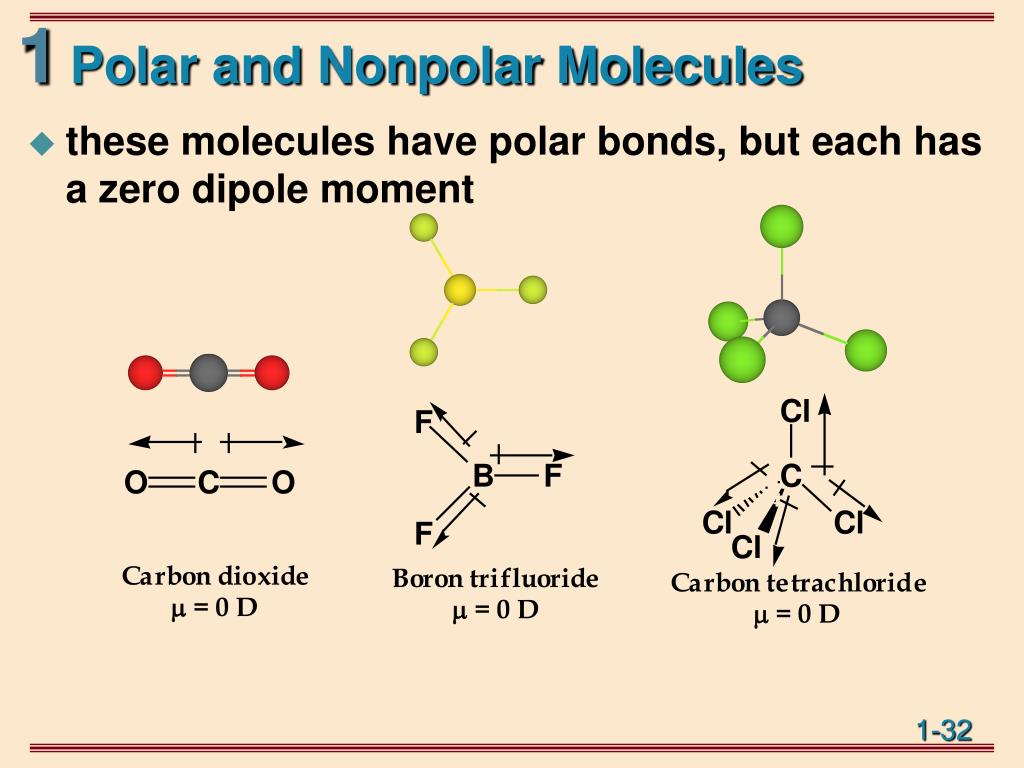
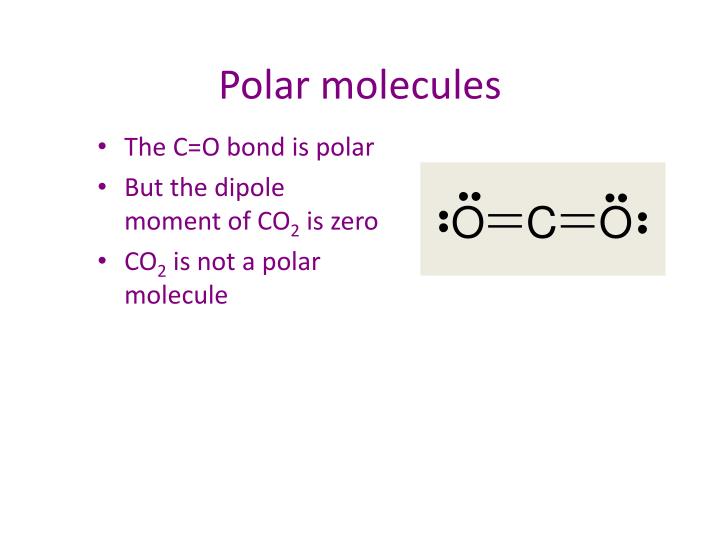
Each C-O bond in CO 2 is polar, yet experiments show that the CO 2 molecule has no dipole moment. Because the two C-O bond dipoles in CO 2 are equal in magnitude and oriented at 180° to each other, they cancel. As a result, the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge.
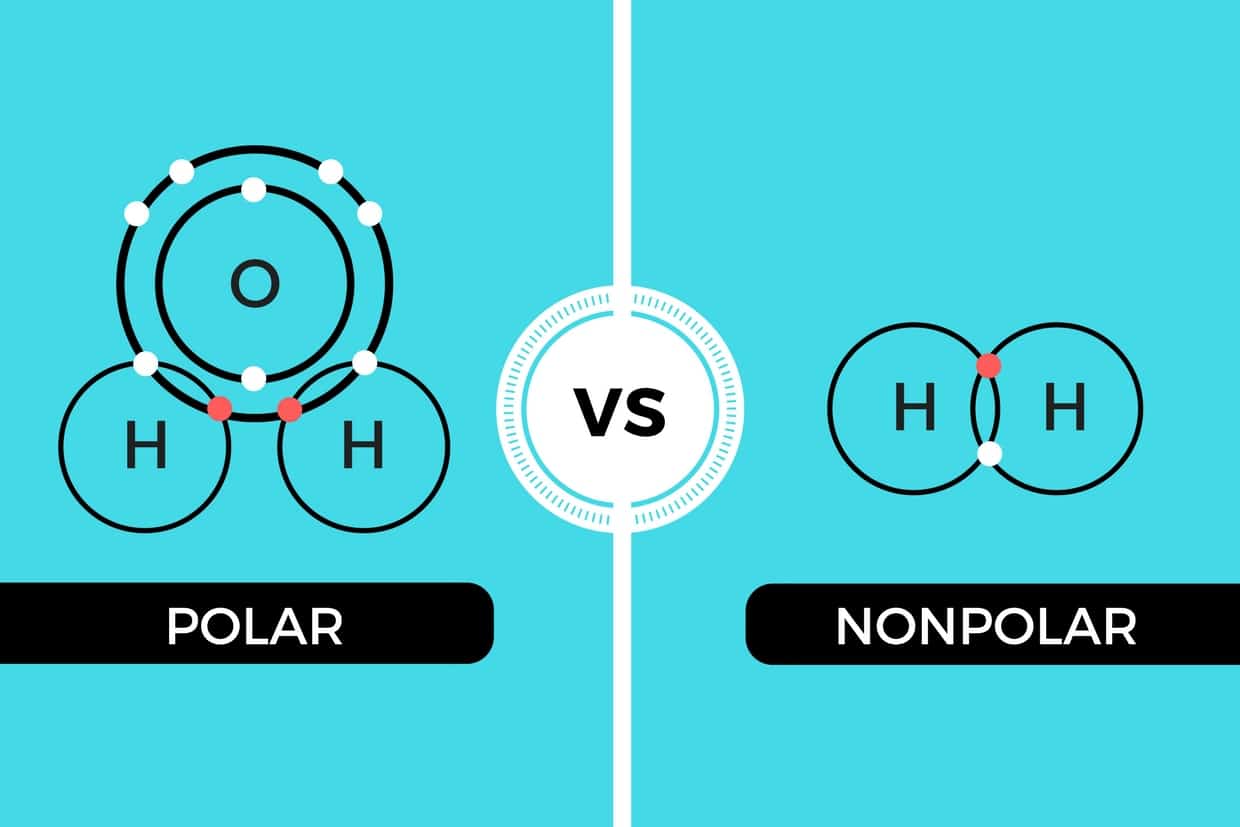
Polar and Nonpolar Covalent Bonds Characteristics & Differences
Want to know the reason? Let's dive into it! COCl2 is a POLAR molecule because the C=O bond and C-Cl bonds present in the molecule are polar and it has asymmetric geometry which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule.

Is SF4 polar or nonpolar [1 Best explanation] Science Education and
If these centers lie at the same point in space, then the molecule has no overall polarity (and is non polar). Figure 3: Charge distrubtions. If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule nonpolar. A molecule can only be polar if the structure of that.
Cómo saber si una molécula es polar o no polar La Ciencia y la
Is OCl2 polar or nonpolar? Question = Is OCl2 polar or nonpolar? Answer = OCl2 is Polar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Ppt Polar Bonds And Molecules Powerpoint Presentation, Free Download FD6
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
PPT Lewis Structures PowerPoint Presentation ID5585056
OCl2 is the molecular formula of dichlorine monoxide which is an inorganic compound existing as a brown-yellow gas at room temperature. As dichlorine monoxide belongs to the chlorine oxide family, it is soluble in both water and organic solvents.